Early life is a critical, time-limited period during which microbial colonization at mucosal surfaces exerts a durable influence on immune development, creating a “window of opportunity” in immune education. Preterm birth, defined as birth before 37 weeks of gestation, is the leading cause of neonatal and childhood death globally. Preterm infants are predisposed to prenatal immune activation and inflammation, and preterm birth is associated with microbial dysbiosis of the maternal vaginal tract as well as infant meconium, or first stool of infants. Thus, preterm birth is one of the most critical early life to shape the neonatal immune system with consequences for immune health later in life.
We previously identified a novel effector T cell population in the healthy prenatal intestine which associates with the presence of viable bacteria. Bacterial strains isolated from prenatal meconium exhibited species-specific capacity to modulate the behavior of intestinal T cells, suggesting that the window of immune “receptiveness” to microbial education is not limited to childhood and extends earlier into the womb. Understanding patterns of immune dysregulation and microbial perturbations during this critical window of development is fundamental to elucidating mechanisms of inflammatory disease pathogenesis in preterm infants and understanding their contribution to neonatal outcomes.
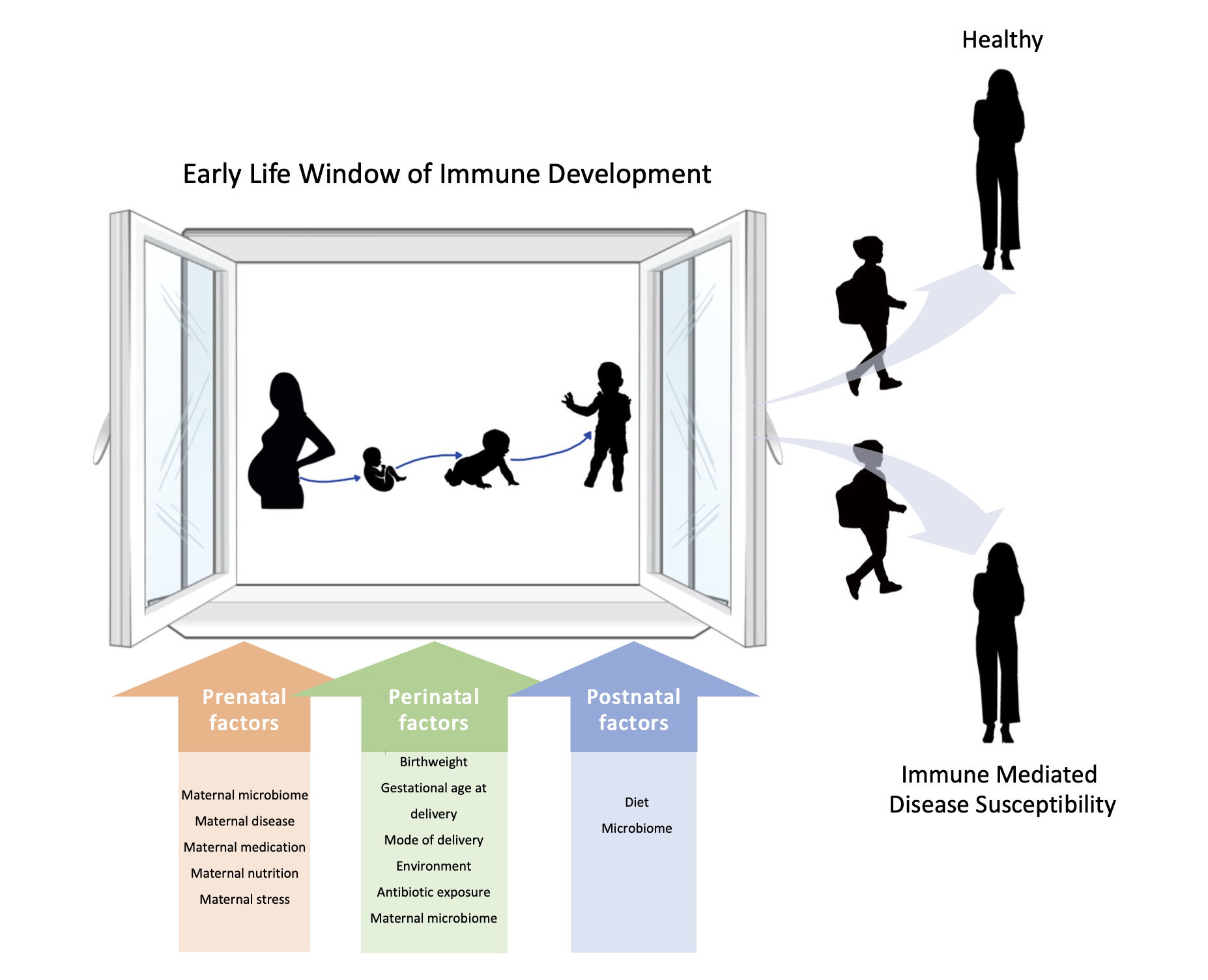
Ongoing areas of research in our lab:
- Identify the mechanisms that instruct the development and regulation of prenatal protective immunity.
- Define patterns of neonatal immune dysregulation and alterations in microbial colonization in preterm birth.
- Characterize the influence of early life host-microbe interactions on human immune development.
We utilize immune and microbial transcriptomics, high parameter cytometry, and in vivo models to decipher the signals that instruct human immune cells during early life.